
Real-world data and real-world evidence are used to monitor the post-market safety and adverse events of drugs. The monitoring of this data assists in making regulatory decisions. The healthcare community is using RWE and RWD to support coverage decisions and to develop guidelines and decision support tools for use in clinical practice.
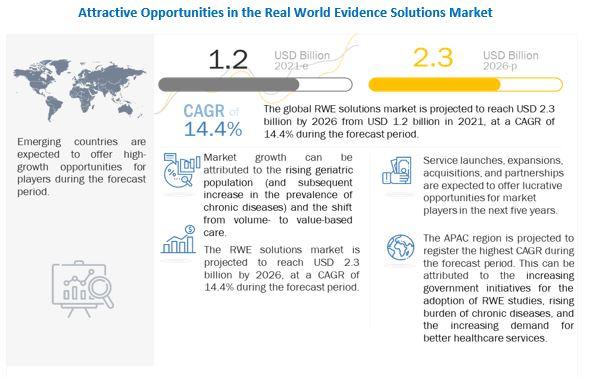
The global RWE solutions market is projected to reach USD 2.3 billion by 2026 from USD 1.2 billion in 2021, at a CAGR of 14.4% during the forecast period. The rising geriatric population (and the subsequent increase in the prevalence of chronic diseases) is a key factor driving the growth of this market.
The shift from volume- to value-based care, delays in drug development (and the subsequent increase in development costs), growth in R&D spending, and support from regulatory bodies for the use of RWE solutions are some of the other major factors that are driving the growth of this market.
RWE is set to become the most influential emerging technology to help in the fight against the COVID-19 outbreak, according to the latest poll on Global Data’s Pharmaceutical Technology website. In this poll, which was completed by 935 of its readers in April 2021, more than one-third of the respondents indicated that RWE would have the greatest impact on the management of COVID-19.
For More Info, Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=76173991
Even though emerging technologies, such as telemedicine, have existed for decades, most of healthcare systems rely heavily on in-person interactions between patients and clinicians. Nevertheless, the current requirement for social distancing measures is swiftly pushing the primary care provision toward remote care.
Telemedicine and virtual care may also prompt a greater adoption of technologies such as wearables and digital therapeutics, thus accelerating digitalization in the healthcare space and boosting the importance of RWE and AI.
Regulators use RWE to monitor the safety of marketed products through traditional pharmacovigilance tools (for instance, Periodic Benefit-Risk Evaluation Report, Periodic Safety Update Report, and Vaccine Adverse Event Reporting System) as well as newer digital aids such as the FDA Sentinel Initiative, a post-market active safety surveillance system.
Get Free Sample Pages @ https://www.marketsandmarkets.com/requestsampleNew.asp?id=76173991



























