
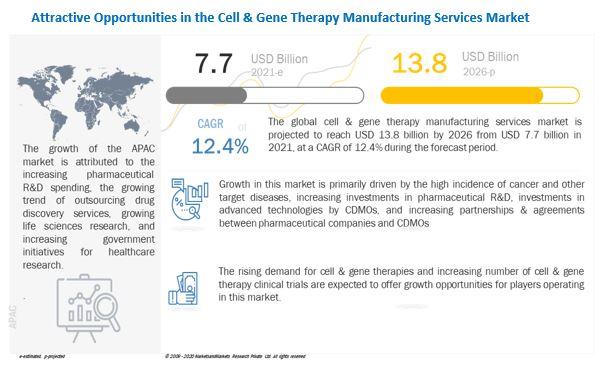
[299 Pages Report] The global cell & gene therapy manufacturing services market size is projected to reach USD 13.8 billion by 2026 from USD 7.7 billion in 2021, at a CAGR of 12.4% during the forecast period. Growth in this market is primarily driven by the high incidence of cancer and other target diseases, increasing investments in pharmaceutical R&D, investments in advanced technologies by CDMOs, and increasing partnerships & agreements between pharmaceutical companies and CDMOs.
The increase in pharmaceutical R&D has resulted in a sharp increase in the number of cell & gene therapy candidates under development. This has made it necessary to outsource manufacturing services to develop cost-effective and efficient cell & gene therapies.
Most pharmaceutical companies continue to invest heavily in the development of novel drugs and devices. The pharmaceutical industry, in particular, is R&D-intensive. Pharmaceutical companies invest in R&D to deliver high-quality and innovative products to the market. The trend suggests that the top pharma companies are increasing their R&D efficiencies through heavy R&D investments to see returns on their investment in the long run and through collaborative R&D efforts.
Clinical trials are the backbone of medical research and help pharmaceutical and biopharmaceutical companies develop and commercialize new cell & gene therapies. In the past few years, the demand for clinical trials has risen worldwide as a result of the growing demand for novel medicines to fulfill unmet medical needs.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=180609441
According to a 2020 PhRMA report on the cell & gene therapy pipeline in 2018, there were 289 cell & gene therapies in clinical development by biopharmaceutical companies. This number increased by 25% in 2020, with 362 cell & gene therapies in clinical development. In addition to this, according to data released by CGT Catapult, there were 154 ATMP clinical trials ongoing in the UK in 2020 compared to the 127 trials reported in 2019, indicating an increase of more than 20%.
On the basis of type, the cell and gene therapy manufacturing services is broadly segmented into cell therapy and gene therapy. In 2020, cell therapy accounted for the largest share of the cell & gene therapy manufacturing services market.



























